Note
This page was generated from tutorials/chemistry/06_calculating_thermodynamic_observables.ipynb.
Run interactively in the IBM Quantum lab.
Calculating Thermodynamics Observables with a quantum computer¶
[1]:
# imports
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
from functools import partial
from qiskit.aqua import QuantumInstance
from qiskit import BasicAer
from qiskit.aqua.algorithms import NumPyMinimumEigensolver, VQE, IQPE
from qiskit.aqua.components.optimizers import SLSQP
from qiskit.chemistry.components.initial_states import HartreeFock
from qiskit.circuit.library import ExcitationPreserving
from qiskit.circuit.library import ExcitationPreserving
from qiskit import BasicAer
from qiskit.aqua.algorithms import NumPyMinimumEigensolver, VQE
from qiskit.aqua.components.optimizers import SLSQP
from qiskit.chemistry.components.initial_states import HartreeFock
from qiskit.chemistry.components.variational_forms import UCCSD
from qiskit.chemistry.drivers import PySCFDriver, UnitsType
from qiskit.chemistry.core import TransformationType, QubitMappingType
from qiskit.aqua.algorithms import VQAlgorithm, VQE, MinimumEigensolver
from qiskit.chemistry.transformations import FermionicTransformation
from qiskit.chemistry.drivers import PySCFDriver
from qiskit.chemistry.algorithms.ground_state_solvers import GroundStateEigensolver
from qiskit.chemistry.algorithms.pes_samplers.extrapolator import *
from qiskit.chemistry.algorithms.pes_samplers.bopes_sampler import BOPESSampler
from qiskit.chemistry.drivers.molecule import Molecule
import qiskit.chemistry.constants as const
from qiskit.chemistry.algorithms.pes_samplers.potentials.energy_surface_spline import EnergySurface1DSpline
from thermodynamics_utils.thermodynamics import constant_volume_heat_capacity
from thermodynamics_utils.vibronic_structure_fd import VibronicStructure1DFD
from thermodynamics_utils.partition_function import DiatomicPartitionFunction
from thermodynamics_utils.thermodynamics import Thermodynamics
import warnings
warnings.simplefilter('ignore', np.RankWarning)
/home/computertreker/git/qiskit/qiskit-dup/.tox/docs/lib/python3.7/site-packages/pyscf/lib/misc.py:47: H5pyDeprecationWarning: Using default_file_mode other than 'r' is deprecated. Pass the mode to h5py.File() instead.
h5py.get_config().default_file_mode = 'a'
A preliminary draft with more information related to this tutorial can be foung in preprint: Stober et al, arXiv 2003.02303 (2020)
Calculation of the Born Openheimer Potenitial Energy Surface (BOPES)¶
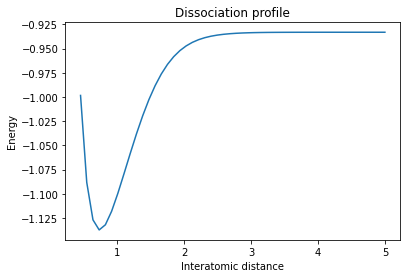
To compute thermodynamic observables we begin with single point energy calculation which calculates the wavefunction and charge density and therefore the energy of a particular arrangement of nuclei. Here we compute the Born-Oppenheimer potential energy surface of a hydrogen molecule, as an example, which is simply the electronic energy as a function of bond length.
[2]:
ft = FermionicTransformation()
driver = PySCFDriver()
solver = VQE(quantum_instance=
QuantumInstance(backend=BasicAer.get_backend('statevector_simulator')))
me_gss = GroundStateEigensolver(ft, solver)
[3]:
stretch1 = partial(Molecule.absolute_distance, atom_pair=(1, 0))
mol = Molecule(geometry=[('H', [0., 0., 0.]),
('H', [0., 0., 0.2])],
degrees_of_freedom=[stretch1],
masses=[1.6735328E-27, 1.6735328E-27]
)
# pass molecule to PSYCF driver
driver = PySCFDriver(molecule=mol)
[4]:
# BOPES sampler testing
bs = BOPESSampler(gss=me_gss,
bootstrap=True)
points = np.linspace(0.45, 5, 50)
res = bs.sample(driver,points)
[5]:
energies = []
bs_res_full = res.raw_results
for point in points:
energy = bs_res_full[point]['computed_energies'][0] + bs_res_full[point]['nuclear_repulsion_energy']
energies.append(energy)
[6]:
fig = plt.figure()
plt.plot(points,energies)
plt.title('Dissociation profile')
plt.xlabel('Interatomic distance')
plt.ylabel('Energy')
[6]:
Text(0, 0.5, 'Energy')
[7]:
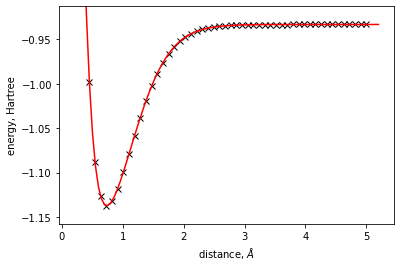
energy_surface = EnergySurface1DSpline()
xdata = res.points
ydata = res.energies
energy_surface.fit(xdata=xdata, ydata=ydata)
[8]:
plt.plot(xdata, ydata, 'kx')
x = np.arange(min(xdata) - 0.25, max(xdata) + 0.25, 0.05)
plt.plot(x, energy_surface.eval(x), 'r-')
plt.xlabel(r'distance, $\AA$')
plt.ylabel('energy, Hartree')
dist = max(ydata) - min(ydata)
plt.ylim(min(ydata) - 0.1 * dist, max(ydata) + 0.1 * dist)
[8]:
(-1.157682236907866, -0.9127522789218231)
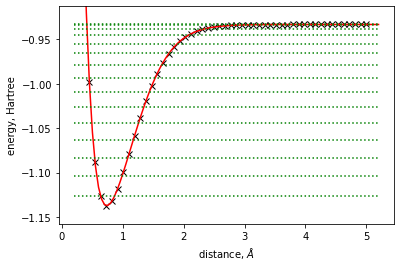
Calculation of the molecular Vibronic Energy levels¶
Here we fit a “full” energy surface using a 1D spline potential and use it to evaluate molecular vibrational energy levels.
[9]:
vibronic_structure = VibronicStructure1DFD(mol, energy_surface)
plt.plot(xdata, ydata, 'kx')
x = np.arange(min(xdata) - 0.25, max(xdata) + 0.25, 0.05)
plt.plot(x, energy_surface.eval(x), 'r-')
plt.xlabel(r'distance, $\AA$')
plt.ylabel('energy, Hartree')
dist = max(ydata) - min(ydata)
plt.ylim(min(ydata) - 0.1 * dist, max(ydata) + 0.1 * dist)
for N in range(15):
on = np.ones(x.shape)
on *= (energy_surface.eval(energy_surface.get_equilibrium_geometry()) +
vibronic_structure.vibrational_energy_level(N))
plt.plot(x, on, 'g:')
on = np.ones(x.shape)
plt.show()
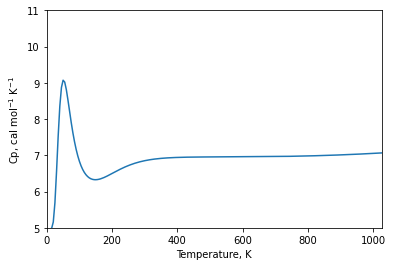
Create a partition function for the calculation of heat capacity¶
The partition function for a molecule is the product of contributions from translational, rotational, vibrational, electronic, and nuclear degrees of freedom. Having the vibrational frequencies, now we can obtain the vibrational partition function \(q_{\rm vibration}\) to compute the whole molecular partition function \begin{equation} q_{\rm vibration} = \prod_{i=1} ^M \frac{\exp\,(-\Theta_{\nu_i}/2T)}{1-\exp\,(-\Theta_{\nu_i}/2T} \, . \end{equation} Here \(\Theta_{\nu_i}= h\nu_i/k_B\), \(T\) is the temperature and \(k_B\) is the Boltzmann constant.
The single-point energy calculations and the resulting partition function can be used to calculate the (constant volume or constant pressure) heat capacity of the molecules. The constant volume heat capacity, for example, is given by
\begin{equation} C_v = \left.\frac{\partial U}{\partial T}\right|_{N,V}\, , \qquad {\rm with} \quad U=k_B T^2 \left.\frac{\partial {\rm ln} Q}{\partial T}\right|_{N,V} . \end{equation}
\(U\) is the internal energy, \(V\) is the volume and \(Q\) is the partition function.
Here we illustrate the simplest usage of the partition function, namely creating a Thermodynamics object to compute properties like the constant pressure heat capacity defined above.
[10]:
Q = DiatomicPartitionFunction(mol, energy_surface, vibronic_structure)
P = 101350 # Pa
temps = np.arange(10, 1050, 5) # K
mol.spins = [1/2,1/2]
td = Thermodynamics(Q, pressure = 101350)
td.set_pressure(101350)
temps = np.arange(10, 1500, 5)
ymin = 5
ymax = 11
plt.plot(temps,
td.constant_pressure_heat_capacity(temps) / const.CAL_TO_J)
plt.xlim(0, 1025)
plt.ylim(ymin, ymax)
plt.xlabel('Temperature, K')
plt.ylabel('Cp, cal mol$^{-1}$ K$^{-1}$')
plt.show()
Here we demonstrate how to access particular components (the rotational part) of the partition function, which in the H2 case we can further split to para-hydrogen and ortho-hydrogen components.
[11]:
eq = Q.get_partition(part="rot", split="eq")
para = Q.get_partition(part="rot", split="para")
ortho = Q.get_partition(part="rot", split="ortho")
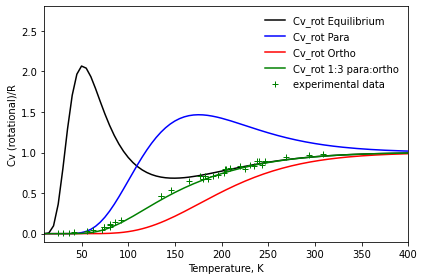
We will now plot the constant volume heat capacity (of the rotational part) demonstrating how we can call directly the functions in the ‘thermodynamics’ module, providing a callable object for the partition function (or in this case its rotational component). Note that in the plot we normalize the plot dividing by the universal gas constant R (Avogadro’s number times Boltzman’s constant) and we use crossed to compare with experimental data found in literature.
[12]:
# REFERENCE DATA from literature
df_brink_T = [80.913535,135.240157,176.633783,219.808499,246.226899]
df_brink_Cv = [0.118605,0.469925,0.711510,0.833597,0.895701]
df_eucken_T = [25.120525, 30.162485, 36.048121, 41.920364, 56.195875, 62.484934, 72.148692, 73.805910, 73.804236, 92.214423,180.031917,230.300866]
df_eucken_Cv = [0.012287,0.012354,0.008448,0.020478,0.032620,0.048640,0.048768,0.076678,0.078670,0.170548,0.667731,0.847681]
df_gia_T = [190.919338,195.951254,202.652107,204.292585,209.322828,225.300754,234.514217,243.747768]
df_gia_Cv = [0.711700,0.723719,0.749704,0.797535,0.811546,0.797814,0.833793,0.845868]
df_parting_T = [80.101665, 86.358919,185.914204,239.927797]
df_parting_Cv = [0.084730,0.138598,0.667809,0.891634]
df_ce_T = [80.669344,135.550569,145.464190,165.301153,182.144856,203.372528,237.993108,268.696642,294.095771,308.872014]
df_ce_Cv = [0.103048,0.467344,0.541364,0.647315,0.714078,0.798258,0.891147,0.944848,0.966618,0.985486]
[13]:
HeatCapacity = constant_volume_heat_capacity
R = const.N_A * const.KB_J_PER_K
plt.plot(temps,
HeatCapacity(eq, temps) / R, '-k',
label='Cv_rot Equilibrium')
plt.plot(temps, HeatCapacity(para, temps) / R, '-b',
label='Cv_rot Para')
plt.plot(temps, HeatCapacity(ortho, temps) / R, '-r',
label='Cv_rot Ortho')
plt.plot(temps, 0.25 * HeatCapacity(para, temps) / R
+ 0.75 * HeatCapacity(ortho, temps) / R, '-g',
label='Cv_rot 1:3 para:ortho')
plt.plot(df_brink_T, df_brink_Cv, '+g')
plt.plot(df_eucken_T, df_eucken_Cv, '+g')
plt.plot(df_gia_T, df_gia_Cv, '+g')
plt.plot(df_parting_T, df_parting_Cv, '+g')
plt.plot(df_ce_T, df_ce_Cv, '+g', label = 'experimental data')
plt.legend(loc='upper right', framealpha=100, frameon=False)
plt.xlim(10, 400)
plt.ylim(-0.1, 2.8)
plt.xlabel('Temperature, K')
plt.ylabel('Cv (rotational)/R')
plt.tight_layout()
plt.show()
[14]:
import qiskit.tools.jupyter
%qiskit_version_table
%qiskit_copyright
Version Information
| Qiskit Software | Version |
|---|---|
| Qiskit | 0.24.1 |
| Terra | 0.16.4 |
| Aer | 0.7.6 |
| Ignis | 0.5.2 |
| Aqua | 0.8.2 |
| IBM Q Provider | 0.12.2 |
| System information | |
| Python | 3.7.7 (default, Apr 22 2020, 19:15:10) [GCC 9.3.0] |
| OS | Linux |
| CPUs | 32 |
| Memory (Gb) | 125.71903228759766 |
| Tue May 25 15:12:35 2021 EDT | |
This code is a part of Qiskit
© Copyright IBM 2017, 2021.
This code is licensed under the Apache License, Version 2.0. You may
obtain a copy of this license in the LICENSE.txt file in the root directory
of this source tree or at http://www.apache.org/licenses/LICENSE-2.0.
Any modifications or derivative works of this code must retain this
copyright notice, and modified files need to carry a notice indicating
that they have been altered from the originals.